- Home
- Are all products containing hydroabsorbent polymers the same?
Are all products containing hydroabsorbent polymers the same?
01/03/2021 - 00:00
Hydroabsorbent, water-absorbing or superabsorbent polymers (SAP), hydrogels or superabsorbers… although on the exterior they may look similar to another, their chemical structure can be vastly different.
It is that chemical construction, thus physical structure of the network and density, that will affect how they absorb, store and release their contents. Furthermore, the cross linking of the long polymer molecules into a three-dimensional matrix will determine their toxicity, longevity and suitability for their use in growing plants.
A short chemistry lesson…
Hydroabsorbent polymers are made from the polymerization of acrylic acids which are transformed into poly-acrylic acid salts. Another material used to make superabsorbent polymers is polyacrylamide. Salts typically are ionic assemblies of related numbers cations (positively charged ions) and anions (negatively charged ions) so that the product is electrically neutral (without a net charge).
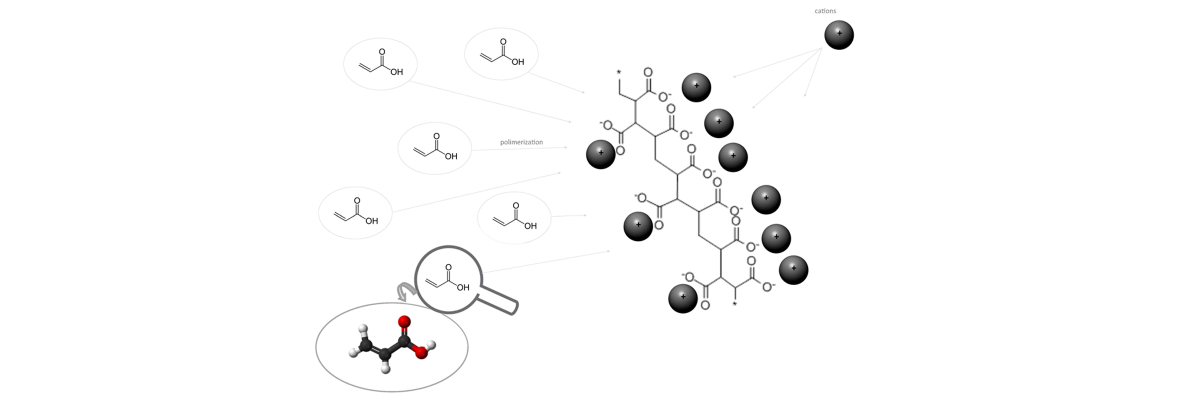
| Polymerization of acrylic acid into poly-acrylic acid salts. |
These cations, needed to neutralise the electrical charge of the polymer, largely determine the properties of the molecule:
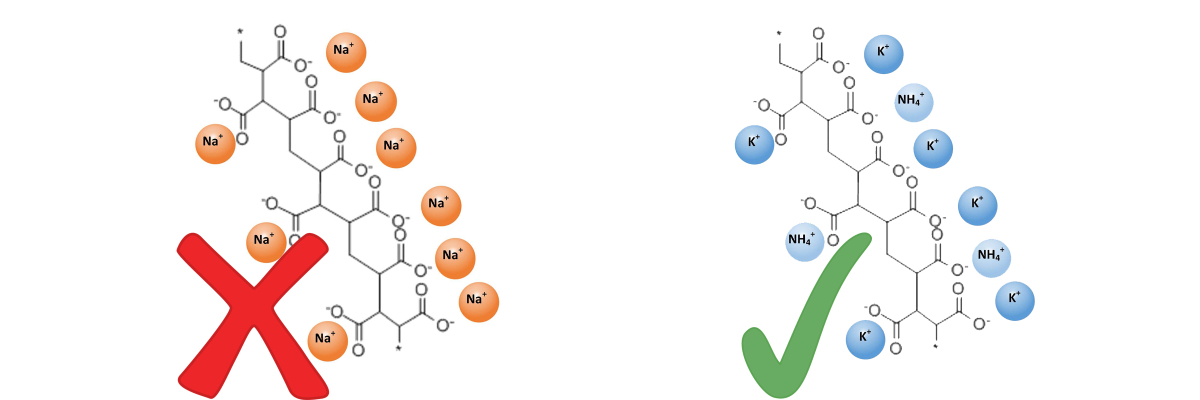
- Sodium-based poly-acrylic acid salts, sometimes referred to as sodium polyacrylate (or PAA), are manufactured principally for use in baby diapers and other sanitary ware, for use as flocculants and for chemical liquid waste disposal, making them unsuitable for use with plants or turf. Unfortunately, some of these products are repackaged and sold for use in horticulture.
- Only polymers that are of salts of potassium and/or ammonium are suitable for agricultural and horticultural uses.
|
Sodium-based poly-acrylic acid salts are manufactured for industrial purposes (baby diapers, sanitary ware, flocculants, etc.) making them UNSUITABLE for use with plants or turf. |
Screening
Furthermore, the TerraCottem soil conditioning technology simply contains those hydroabsorbent products that have demonstrated the best results on plant growth and water efficiency, in independent efficacy trials carried out by and/or on behalf of TerraCottem.
If you know someone who would also like to read this article, feel free to share:
Take me back to the Frequently Asked Questions
We are happy to answer any further questions:
continue to our webformWe are happy to answer any further questions:
continue to our webformTerraCottem Intl. SL
Apartado de Correos 4511190 Benalup (Cádiz)Spain

